The Value of Sovaldi: Societal Cost Issues of New Interventions on Hepatitis C

The Value of Sovaldi: Societal Cost Issues of New Interventions on Hepatitis C
September 2014 | White Paper
To view the full report, please click on the "download" button to receive a full PDF copy.
Key Insight
“Value plays a pivotal role in policy prescriptions regarding the allocation of financial resources to medical care. Costs and benefits from treatment must be considered to accurately assess the true value of drug interventions to society."
Introduction
Welcome to this Center for Healthcare Innovation (CHI) white paper: The Value of Sovaldi: Societal Cost Issues of New Interventions on Hepatitis C. In December 2013, the FDA approved a new drug, sofosbuvir, for the treatment of Chronic Hepatitis C. The drug, commonly known as Sovaldi, was developed by Gilead Sciences, a U.S. biotechnology company. The initial price tag of a 12- week treatment of Sovaldi was reported as approximately $84,000, or nearly $1,000 per pill. This resulted in considerable media attention and an ensuing pricing controversy. Editorials and op-eds sprung up around the country debating Sovaldi’s price tag and the broader debate over fair drug pricing. Some politicians expressed outrage over the cost of new drugs, while others argued for free-market pricing and rewards for the considerable R&D costs that biopharmaceutical companies incur when bringing a new drug to market. The often niche drug pricing debate had officially spilled over into the mainstream conversation. Payers, policymakers, pharma, patients, and providers all voiced strong— and sometimes contrasting— opinions.
At CHI, we aim to help these stakeholders increase their knowledge and understanding of healthcare value, which we view as a function of quality, access, and cost. Thus, we decided to further explore the value of Sovaldi and how the costs and benefits of the drug relate to the broader discussion of the treatment of Hepatitis C. Our goal is to offer a more informed and analytical approach to the discussion of the value of Sovaldi. One question that immediately arose was “How does the price of Sovaldi compare to the long-term costs of the treatment of Chronic Hepatitis C?” Our goal was to analyze the complex interrelationships and broader macroeconomic principles relating to the costs and benefits of a drug, as well as the long-term costs of treating Chronic Hepatitis C.
By analyzing the societal cost implications of Chronic Hepatitis C, we aim to help patients, providers, pharma, pharmacy, payers, and policymakers increase their knowledge and understanding of the value of this treatment—as well as the complex relationships between drug costs and the longer term costs of a disease. We hope that you find this white paper to be both thought-provoking and useful, and we welcome your feedback. We thank you for your interest, and we hope you enjoy our comprehensive analysis.
PART 1: Hepatitis C Background
Decision-makers in these organizations are tasked with determining who covers costs, the degree of financial resources for treatment, and whether the benefits of treatment outweigh economic costs to society. These decisions are central to the drug pricing conversation. At issue is the justification of drug pricing based on remuneration for millions of dollars in research costs and the criterion that delineates reasonable profit from outright extortion. Answers will shape the nexus of healthcare drug utilization and resource allocation for patients, pharmaceutical companies, hospitals, taxpayers, and insurance and regulatory agencies.
The figures surrounding Sovaldi’s public debate are sobering. The Kaiser Family Foundation estimates that if the 3 million Americans infected with HCV were to be treated with Sovaldi at an average cost of $100,000 per treatment, the amount the U.S. spends on prescription drugs in one year would double from $300 billion to $600 billion (Appleby, 2014). The costs of specialty drugs like Sovaldi are expected to quadruple to $400 billion by 2020, which would account for 9.1% of national health spending (UnitedHealth Center for Health Reform & Modernization, 2014). The confluence of increasing drug costs and limited resources may elicit contentious topics such as determining who should receive treatment. Should those in taxpayer-funded programs have the same access as the sickest patients? Should the severity of disease dictate allocation priorities? The American Association for the study of Liver Diseases and the Infectious Diseases of Society believe Sovaldi should be the preferred treatment for all who are viremic. The Department of Veterans Affairs, however, argues for a treatment reserved only for those with advanced liver disease, a complication of chronic HCV infection. Currently, 47 states provide limited coverage of Sovaldi for their Medicaid populations. Half these states implement policies of “prior authorization” that require a patient be in the worst stages of hepatitis C for eligibility. States practicing these policies are those with the largest Medicaid populations and the most patients who suffer from HCV. Evaluating the costs of HCV treatment equips policymakers with the knowledge to enact policies that engender the best health outcomes at the “right” price.
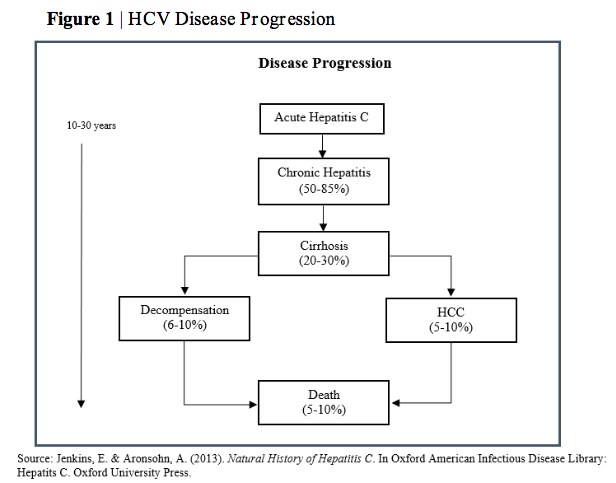
Key Statistics and Infographics
To view the full report, please click on the "download" button to receive a full PDF copy.
Authors
Corey Mason, MS
Senior Analyst at CHI
Joseph Gaspero
CEO & Co-Founder at CHI
Dr. Stuart Rich, MD
Special Fellow at CHI
Professor of Medicine at Northwestern Medicine
Joseph Gaspero is the CEO and Co-Founder of CHI. He is a healthcare executive, strategist, and researcher. He co-founded CHI in 2009 to be an independent, objective, and interdisciplinary research and education institute for healthcare. Joseph leads CHI’s research and education initiatives focusing on including patient-driven healthcare, patient engagement, clinical trials, drug pricing, and other pressing healthcare issues. He sets and executes CHI’s strategy, devises marketing tactics, leads fundraising efforts, and manages CHI’s Management team. Joseph is passionate and committed to making healthcare and our world a better place. His leadership stems from a wide array of experiences, including founding and operating several non-profit and for-profit organizations, serving in the U.S. Air Force in support of 2 foreign wars, and deriving expertise from time spent in industries such as healthcare, financial services, and marketing. Joseph’s skills include strategy, management, entrepreneurship, healthcare, clinical trials, diversity & inclusion, life sciences, research, marketing, and finance. He has lived in six countries, traveled to over 30 more, and speaks 3 languages, all which help him view business strategy through the prism of a global, interconnected 21st century. Joseph has a B.S. in Finance from the University of Illinois at Chicago. When he’s not immersed in his work at CHI, he spends his time snowboarding backcountry, skydiving, mountain biking, volunteering, engaging in MMA, and rock climbing.